Abstract
Background: Paget-Schroetter Syndrome (PSS), also known as venous thoracic outlet syndrome or effort-induced upper extremity deep vein thrombosis, is a rare condition that occurs secondary to impingement of the subclavian vein by the overlying cervical ribs, long transverse processes of the cervical spine, musculo-fascial bands, or clavicular or first rib abnormalities. PSS often affects otherwise healthy and athletic individuals with a history of repetitive overhead activities. Given the paucity of published pediatric data, making evidence-based recommendations on appropriate therapeutic strategies is challenging.
Objective: The principal objective of this single-institution study was to review the presentation, management, and outcomes of pediatric patients treated for PSS at Nationwide Children's Hospital (NCH) over a six-year period (January 1, 2010 to December 31, 2016).
Methods: The study was approved by the Institutional Review Board at NCH. The Electronic Data Warehouse was used to identify patients diagnosed with PSS during the 6-year study period using modified ICD-9-CM codes. Eligible subjects were defined as children under the age of 21 who presented with an unprovoked upper extremity deep vein thrombosis (DVT) and had evidence of compression of the subclavian vein at the level on the thoracic outlet on dynamic imaging. Baseline demographic data, diagnostic and therapeutic details, and available follow-up information was abstracted from patient charts. Eligible subjects were also mailed a previously validated pediatric post-thrombotic syndrome (PTS) self-report instrument and a self-report health-related quality of life (HRQoL) instrument (PedsQL 4.0). All data were summarized and presented using descriptive statistics. Comparisons were made using nonparametric statistical methods.
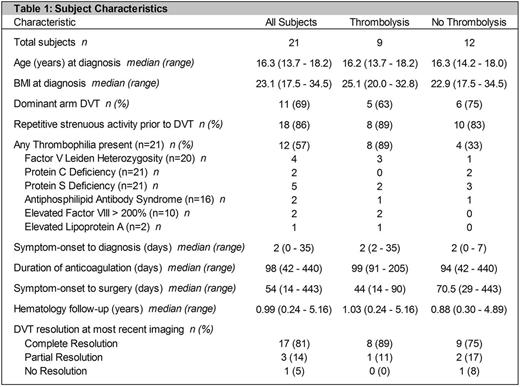
Results: The study cohort consisted of 21 subjects (11 female). Median age at DVT diagnosis was 16.3 (range 13.7-18.2) years. Subjects presented to NCH a median of 2 (range 0-34) days after symptom onset. Eighteen subjects (86%) reported repetitive exercise or overuse activity prior to diagnosis. Twelve subjects (57%) had evidence of congenital/acquired thrombophilia (Table 1). All subjects were treated with anticoagulation for a median duration of 3.2 (range 1.4-8.6) months. Nine subjects (43%) were also treated with catheter-directed and/or pharmaco-mechanical thrombolysis, and six of these subjects additionally underwent balloon angioplasty. All 21 subjects underwent decompressive surgery (first or cervical rib resection) a median of 7.7 (range 2-63.2) weeks after symptom onset. Four subjects (19%) experienced surgical complications, including minor bleeding/hematoma formation (3), pneumothorax (3), and winged scapula (1). Only 2/14 subjects diagnosed before 2014 underwent thrombolytic therapy, whereas all (7/7) subjects diagnosed after 2014 underwent thrombolysis. The subjects who did not undergo thrombolysis were not significantly different from those who did, except for the presence of a thrombophilia (p=0.02). Of the twelve subjects who did not undergo thrombolysis, eleven (92%) had complete/partial resolution of their DVT and one (8%) had no resolution on most recent imaging. Of the nine subjects who received thrombolysis, 100% had complete/partial resolution of their DVT on most recent imaging. We are currently analyzing data on PTS and HRQoL.
Discussion: Herein, we report one of the largest pediatric cohorts of PSS. All subjects received anticoagulation and underwent decompressive surgery. Additionally, 9/21 subjects underwent catheter-directed and/or pharmaco-mechanical thrombolysis. 20/21 subjects had complete/partial resolution of the DVT on most recent imaging. Our single-institution study suggests that high rates of thrombus resolution may be achieved in children with PSS with anticoagulation and decompressive surgery, with or without thrombolytic therapy. Larger, prospective studies are needed to confirm our findings and further examine the relationship between treatment and outcomes.
O'Brien: Bristol Myers Squibb: Other: study of direct oral anticoagulant in prevention of pediatric VTE, Research Funding; Glaxo Smith Kline: Other: DSMB for Arixtra Study in Pediatric VTE; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: advisory board - von Willebrand Disease diagnosis & management; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: advisory board - VWD diagnosis and management; Pfizer: Consultancy, Other: study of direct oral anticoagulant in treatment of pediatric VTE. Kumar: CSL Behring: Consultancy; Bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.